Study explores blood-brain barrier leakage in CNS infections
American Society for Microbiology | 08-06-2019
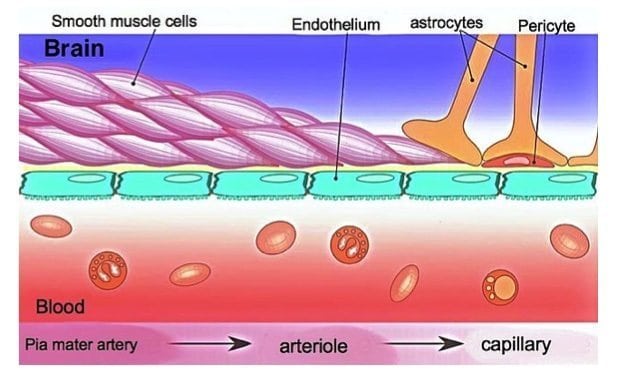

A new study published in the journal mBio shines light on the breakdown of the blood-brain barrier (BBB) that occurs during many infections of the central nervous system. The findings implicate interferon-gamma, a major cytokine upregulated in most central nervous system (CNS) viral infections, as a major contributor of blood-brain barrier breakdown. Using an experimental viral encephalitis mouse model in which mice are infected with reovirus, the research provides new insight into how the breakdown occurs, which may lead to new therapeutic avenues.
“Gene expression studies on brain material from infected mice suggested that one of the pathways that was really upregulated during infection was interferon signaling in general, and in particular, a subset of interferon, the type 2 interferon or interferon-gamma,” said study investigator Kenneth Tyler, MD, a neurovirologist and Chairman of the Department of Neurology at the University of Colorado School of Medicine. “Interferon was one of the things that was causing not only a loss of pericytes, support cells, but a disruption of the connections that are usually pretty tight between endothelial cells in the blood-brain barrier called tight junctions and adherens junctions.”
Many previous studies have demonstrated that the blood-brain barrier breaks down during the process of encephalitis. Mechanistically, there have been some connections between type 1 and type 2 interferon and that process, but what occurs at the cellular level and molecular level, at the level of the vasculature, has been very unclear.
“Is the blood-brain barrier breakdown an early feature of the pathology? Is it late? What sort of relationship does the breakdown have with other aspects of disease progression?” said principal study investigator Julie Siegenthaler, PhD, a neuroscientist in the Department of Pediatrics, University of Colorado School of Medicine.
To find out, scientists at the University of Colorado School of Medicine inoculated reovirus into the brains of neonatal mice, producing devastating encephalitis, which they then closely monitored. The researchers replicated much of what they were seeing in the mouse brains in experiments using cultured brain endothelial cells.
The researchers found that BBB breakdown happens late in the course of the disease. “Disease progression happens over about 10 days. We see the blood-brain barrier breakdown happening in the last 6 to 7 days after infection, after seeing evidence that the virus is being replicated and after seeing evidence that there is an inflammatory response,” said Dr. Siegenthaler.
Infection upregulated interferon-gamma, and endothelial cells in the BBB responded to interferon-gamma by initiating a signaling cascade that changed their behavior so that they could no longer maintain the BBB integrity. IFN-gamma reduced barrier properties in cultured brain endothelial cells through Rho kinase (ROCK) mediated cytoskeletal contractions, resulting in junctional disorganization and cell-cell separation.
“Infection with reovirus causes a change in the proteins that regulate the cytoskeleton,” said Dr. Siegenthaler. “It causes an overactivation of the cytoskeleton, causing the cells to pull apart from each other, which has not been shown before.”
The researchers showed that if they blocked interferon-gamma signaling, by using a neutralizing antibody, many of the disruptions, such as loss of pericytes and changes in tight junctions, could be restored.
The work may be a model for what happens in other forms of viral and even bacterial infections where there is breakdown of the BBB. “We have learned that in a lot of models of both meningitis and viral encephalitis, you need to stop the replication of the pathogen – whether that is an antibiotic for bacterial meningitis or an antiviral when it is available for viral meningitis – and employ strategies that inhibit a whole series of things that add to the seriousness of the disease that occur downstream of that infection.”
“Interferon-gamma seems to be mediating the damage in the endothelial cells that make up the blood vessels,” said Stephanie Bonney, PhD, who is now a researcher at Seattle Children’s Research Institute. “We see changes within the endothelial cells themselves that affect the way they connect to each other. These changes allow for the acceleration of fluids and immune cells into the CNS, contributing to edema and other problems in patients.”
“Manipulating the blood-brain barrier in combination with antiviral therapies may lead to better outcomes,” said Dr. Tyler “You could specifically target the endothelial cells to maintain blood-brain barrier integrity,” said Dr. Siegenthaler.
The scientists say more research is needed to show whether other viruses such as West Nile or another flavivirus behaves similarly to reovirus, and whether the findings can be extrapolated to infections in humans.
Source:
Materials provided by the American Society for Microbiology. Content may be edited for clarity, style, and length.
